 The sun emits different forms of energy in the form of electromagnetic waves. One form is the ultraviolet light which has a shorter wavelength of between 10 to 400 nm than visible light (greater than 400 nm) and a longer wavelength than x-rays (less than 10 nm). Because of its shorter wavelength, ultraviolet light contains higher energy.
The sun emits different forms of energy in the form of electromagnetic waves. One form is the ultraviolet light which has a shorter wavelength of between 10 to 400 nm than visible light (greater than 400 nm) and a longer wavelength than x-rays (less than 10 nm). Because of its shorter wavelength, ultraviolet light contains higher energy.
The ultraviolet light in electromagnetic spectrum is divided into three parts based on their wavelengths and their energies: Near UV, Far UV, and Extreme UV. Near UV has the longest wavelength of the three and therefore, it is the nearest to visible light in the spectrum. Far UV is found between Near and Extreme UV. Extreme UV has the shortest wavelength. The short wavelength means it contains high energy. It is closest to the x-ray in the spectrum.
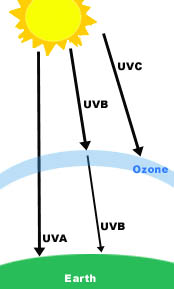
 Ultraviolet light is blocked from entering the earth because of the protective effect of the ozone layer. UV light reacts with ozone so that only safe UV light enters into the earth’s atmosphere. The Near UV gets easily into the atmosphere because of its low energy that it does not react to the ozone. However, the extreme UV almost never goes through the ozone because if binds readily with the molecules of the ozone. Likewise, far UV does not easily get through but small amount are filtered still.
Ultraviolet light is blocked from entering the earth because of the protective effect of the ozone layer. UV light reacts with ozone so that only safe UV light enters into the earth’s atmosphere. The Near UV gets easily into the atmosphere because of its low energy that it does not react to the ozone. However, the extreme UV almost never goes through the ozone because if binds readily with the molecules of the ozone. Likewise, far UV does not easily get through but small amount are filtered still.
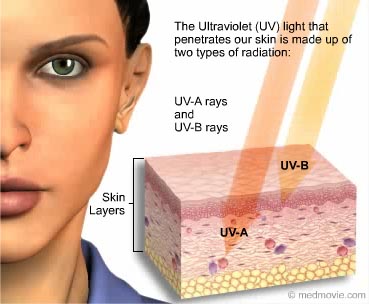
Because of the strength of UV’s energy, it can immediately remove electrons from atoms and cause changes in the molecular make-up of chemicals. It can also definitely cause changes in the cells of the human body.
